
Each oncology drug in our development pipeline goes through numerous phases of rigorous testing and validation to determine efficacy, confirm genomic signatures, and more.
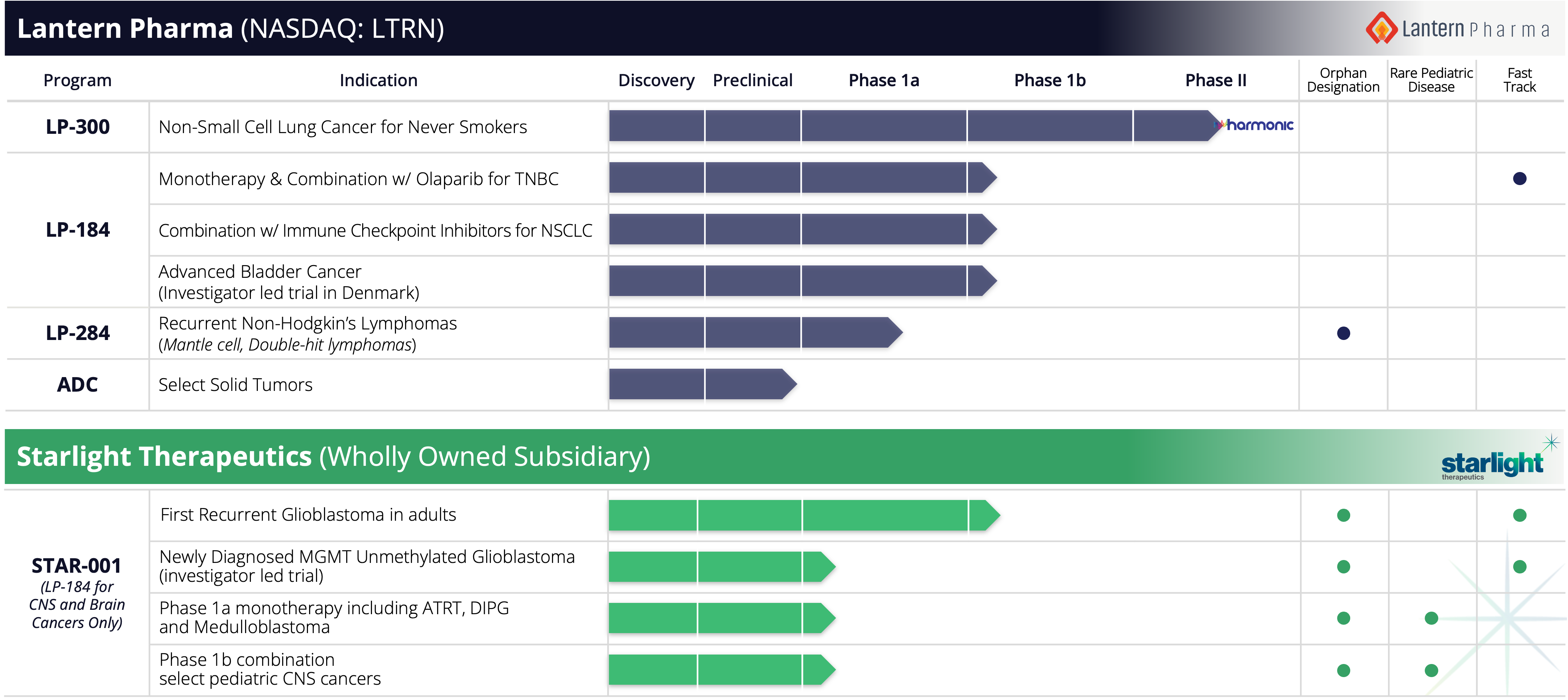
Lantern’s current portfolio consists of three lead drug candidates and an ADC program across 12 cancer indications, including one lead product candidate in phase 2 clinical testing and phase 1 clinical programs for two additional lead product candidates. Lantern believes that the use of machine learning, genomics and computational methods can help accelerate the development and commercialization of small molecule-based therapies. These drugs can be targeted to patients whose genomic profile identifies them as having the highest probability of benefiting from the drug, thereby creating the potential to achieve better outcomes.
.png)

Mechanism of Action
LP-284, is a stereoisomer of LP-184 and a new chemical entity currently in early preclinical R&D stages. It is a distinct pharmaceutical agent with cancer cell-killing activity differing from LP-184 in terms of target indications, potency, metabolism, pharmacokinetics and toxicity. Its mechanism of action is likely related to DNA-damage and DNA-repair inhibition in cancer cells.
Background
LP-284 is a fully synthetic molecule belonging to the new generation of acylfulvenes, a family of naturally-derived anti-cancer drug candidates. It is currently being evaluated for activity in a spectrum of hematological cancers. Together with the work of collaborators, the roadmap to advancement of LP-284 to the clinic includes identification and validation of target indications, response gene signature development leading to IND-enabling pharmacokinetic profiling, tolerability and dose range finding studies followed by clinical protocol development.

Mechanism of Action
LP-184, or hydroxyureamethylacylfulvene, is a small molecule drug candidate currently in preclinical development. It is a next generation alkylating agent that preferentially damages DNA in cancer cells that overexpress certain biomarkers and is from the fulvene class of compounds.
Background
LP-184 is a promising member of a new generation of acylfulvenes, a family of naturally-derived anti-cancer drug candidates. In preclinical studies, LP-184 has shown significantly enhanced anti-tumor activity and substantially reduced toxicity as compared to earlier generation acylfulvenes. In addition, we have used our RADR™ platform together with the work of collaborators, to develop a patient-specific biomarker test predictive of LP-184’s efficacy. We plan on using this test to facilitate patient selection in our planned Phase 1 clinical trial for LP-184.


Mechanism of Action
LP-300, or Disodium 2,2’-dithio-bis-ethane sulfonate, is a small molecule entity with cysteine modifying activity on select proteins. LP-300 works to modulate multiple cellular pathways simultaneously and is a potential first-in-class combination agent indicated in NSCLC.LP-300 shows cysteine modifying activity on select proteins (ALK), and has shown that it modulates protein function (EGFR, MET, and ROS1). It also acts as a chemo-sensitizer for combination therapies by inactivating proteins that are modulating cellular redox status and drug resistance (TRX, GRX) and possesses chemoprotectant activity that reduces toxicities associated with Taxane / Platinum based chemotherapies.
Background
LP-300, originally branded as Tavocept®, is a molecular entity that we believe may be capable of ameliorating the toxic side effects of chemotherapeutic drugs such as cisplatin. It also acts as a chemoenhancer and has been studied in multiple randomized, controlled, multi-center non-small cell lung cancer (NSCLC) trials that included administration of either paclitaxel and cisplatin and/or docetaxel and cisplatin. Retrospective analyses of the results of a multi-country phase III lung cancer trial in subgroups of adenocarcinoma patients receiving LP-300, paclitaxel and cisplatin demonstrated substantial improvement in overall survival, particularly among female non-smokers, where a 13.6 month improvement in overall survival (p-value 0.0167, Hazard Ration 0.367) in favor of LP-300 was observed, as compared to placebo in the subgroup of paclitaxel/cisplatin-treated patients. We are focused on repositioning LP-300 as a potential combination therapy for non-smoking (or never-smoking) female NSCLC patients with histologically defined adenocarcinoma. Currently there is no approved therapy specifically for the growing indication of non-smokers (or never-smoking) with NSCLC, and female non-smokers appear to be uniquely responsive to LP-300. With both chemoprotective and chemosensitizing activity, LP-300 has potential as a combination agent or adjuvant in front line, second line or salvage therapy in newly diagnosed, relapsed, metastatic or advanced NSCLC for overall survival enhancement and toxicity alleviation from primary chemotherapy or standard of care.


Mechanism of Action
LP-100 or 6-hydroxymethylacylfulvene exploits cancer cells’ deficiency in DNA repair mechanisms. LP-100 shows multiple cytotoxic effects on tumor cell biology such as DNA adduct formation, RNA polymerase stalling and redox protein modification. It demonstrates enhanced sensitivity in DNA repair deficient (e.g. ERCC3 mutant or knockout) in vitro and in vivo models. LP-100 leads to rapid inhibition of DNA synthesis and induction of DNA damage. LP-100 is a monofunctional covalent DNA binder that inhibits DNA synthesis and replication, affects cell cycle and induces apoptosis. DNA repair of LP-100-induced lesions is mediated by components of the transcription-coupled nucleotide excision repair (TC-NER) pathway. LP-100 produces damage to DNA that can only be repaired by the TC-NER pathway.
Background
LP-100 was first developed by MGI Pharma and later in collaboration with global pharma company, Eisai. LP-100 has shown strong clinical anti-tumor activity against a 10-12% of patients with multidrug resistant advanced prostate cancer with notable resolution of bone metastases. Lantern’s process was to uncover a genomic signature that determines potential responders, develop suitable preclinical data, and successfully out-license it to Oncology Venture (a European Biotech) in 18 months. LP-100 is currently in an active phase II clinical trial in in AR-targeted and Docetaxel-pretreated metastatic castration-resistant prostate cancer (mCRPC) patients.

LP-300, or Disodium 2,2’-dithio-bis-ethane sulfonate, is a small molecule entity with cysteine modifying activity on select proteins. LP-300 works to modulate multiple cellular pathways simultaneously and is a potential first-in-class combination agent indicated in NSCLC. LP-300 shows cysteine modifying activity on select proteins (ALK), and has shown that it modulates protein function (EGFR, MET, and ROS1). It also acts as a chemo-sensitizer for combination therapies by inactivating proteins that are modulating cellular redox status and drug resistance (TRX, GRX) and possesses chemoprotectant activity that reduces toxicities associated with Taxane / Platinum based chemotherapies.
LP-300, originally branded as Tavocept®, is a late stage investigational new drug that has been studied in multiple randomized, controlled, multi-center non-small cell lung cancer (NSCLC) trials. It has been shown to have chemo-enhancing and nephroprotective effects. The last clinical trial conducted was an international Phase III study in which patients with advanced lung adenocarcinoma received LP-300 in combination with cisplatin and paclitaxel or docetaxel (test arm) or cisplatin and paclitaxel or docetaxel (standard of care/control arm). Although the study did not meet endpoints in the overall patient population, retrospective analyses demonstrated that the addition of LP-300 significantly increased overall survival in the specific subset of never-smoker patients. The overall median survival in never-smokers was 13.2 months for those who received standard of care only compared to 25.2 months (n= 87; p-value= 0.046) for those who received standard of care plus LP-300, representing an increase of 91%. We are now repositioning LP-300 as a potential combination therapy for never-smoking NSCLC patients with histologically defined adenocarcinoma. Currently there is no approved therapy specifically for the growing indication of never-smokers (or non-smokers) with NSCLC. With both chemoprotective and chemosensitizing activity, LP-300 has potential to increase overall survival and alleviate toxicities from primary chemotherapy in front line, second line or salvage therapy in newly diagnosed, relapsed, metastatic or advanced NSCLC.


LP-100 or 6-hydroxymethylacylfulvene exploits cancer cells’ deficiency in DNA repair mechanisms. LP-100 shows multiple cytotoxic effects on tumor cell biology such as DNA adduct formation, RNA polymerase stalling and redox protein modification. It demonstrates enhanced sensitivity in DNA repair deficient (e.g. ERCC3 mutant or knockout) in vitro and in vivo models. LP-100 leads to rapid inhibition of DNA synthesis and induction of DNA damage. LP-100 is a monofunctional covalent DNA binder that inhibits DNA synthesis and replication, affects cell cycle and induces apoptosis. DNA repair of LP-100-induced lesions is mediated by components of the transcription-coupled nucleotide excision repair (TC-NER) pathway. LP-100 produces damage to DNA that can be preferentially repaired by the TC-NER pathway.
LP-100 has the potential to be an important compound — either as monotherapy or in combination — for several challenging cancers that are impacting patients globally. LP-100 is in an existing genomic-signature guided phase 2 clinical trial in Denmark for patients with metastatic castration resistant prostate cancer (mCRPC). 9 patients (out of a targeted enrollment of 27) have been treated in the trial. The median overall survival (OS) for the initial group of 9 patients has been approximately 12.5 months, which is an improvement over other similar fourth-line treatment regimens for mCRPC.
Recent opportunities have also been identified for the potential use of LP-100 in cancers with DNA repair deficiencies, including potential to treat bladder and prostate cancer patients who have a mutation in the ERCC2/3 genes.
There were over 42,900 cases of metastatic castration-resistant prostate cancer in the US during 2020, and over 170,000 cases globally. Approximately 25-30% of these patients in the US and globally, need treatment options in the third-line setting, or later. In addition, approximately 25-30% of all metastatic prostate cancers have been observed to have mutations in DNA repair genes.

LP-184 is synthetically lethal in tumors with defective HR and NER (nucleotide excision repair) pathways. It is an acylfulvene-derived prodrug that is selectively activated in tumors that over-express the oxidoreductase enzyme Prostaglandin Reductase 1 (PTGR1).
LP-184 (hydroxyureamethylacylfulvene) is a small molecule drug candidate that is highly potent and synthetically lethal in cancers harboring defects in multiple DNA damage repair and recombination pathways (HR/NER) and works through multiple mechanisms of action. LP-184 induces DNA damage that requires an intact TC-NER pathway for repair and subsequent cell survival, making it preferentially lethal in tumors with a defective nucleotide excision repair mechanism. LP-184 induced lesions also disrupt DSB repair in DNA making it synthetically lethal in tumors with HRD as well.
LP-184 demonstrated remarkable efficacy against a panel of cell lines, in vivo and ex vivo models validated by in-silico predictions generated by our RADR® A.I. platform.
Lantern Pharma looks forward to further advancing LP-184 as a new, potent treatment option in genetically-defined subsets of patient populations in areas of high unmet clinical need in pancreatic cancers, GBM (including MGMT-unmethylated GBMs, temozolomide-resistant GBMs, and EGFR-aberrant or recurrent GBMs), and brain metastases. LP-184 can target a broad spectrum of cancers such as lung, bladder, breast, prostate, ovarian, liver, head and neck cancers.


LP-284 belongs to the new generation of acylfulvenes, a family of naturally derived anti-cancer drug candidates, and is the stereoisomer (enantiomer) of our drug candidate LP-184. In comparison to our other acylfulvenes, LP-100 and LP-184, LP-284 has distinct anti-tumor activities in a variety of hematological cancers including lymphoma, multiple myeloma, and leukemia. Additional MOA of anti lymphoma activity of LP-284, appears to come from its ability to mute transcription of oncogenic fusion genes and core oncogenic enhancer driven genes such as CCND1 and c-MYC in MCL. LP-284 has the potential to be developed as a monotherapy or combination therapy with other drugs to treat a broad array of hematological cancers.
In early preclinical studies, LP-284 has shown nanomolar potency in several hematological cell lines with the highest potency against all Mantle Cell Lymphoma (MCL) cell lines tested. LP-284 is also being explored for use as a combination therapy with spironolactone. LP-284 significantly delayed tumor progression and prolonged survival of MCL xenograft mouse models as compared to approved agents Ibrutinib and Bortezomib. LP-284 also led to shrinkage and delayed the progression of tumors in pretreated and Ibrutinib or Bortezomib resistant to MCL xenograft tumors. LP-284 is also being explored for use as a combination therapy with spironolactone in a Multiple Myeloma (MM) cell line which enhanced sensitivity towards LP-284 by 2.4 fold. The absence of Ataxia Telangiectasia Mutated (ATM) function in lymphomas and the need for new agents for Relapsed/Refractory Mantle Cell Lymphoma (R/R MCL) warrants further investigation of LP-284.


By clicking “Accept All”, you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts. View our Privacy Policy for more information.

